Acceleration in the field of translational research and regenerative medicine
The application of genome editing technology has facilitated the generation of human disease models not only in mice but also in a variety of other animal species. Among these, rats have attracted increasing attention due to their unique physiological characteristics and the growing body of research demonstrating their advantages in biomedical applications.
To note, rats generally possess a genomic background and anatomical features more similar to those of humans compared to mice. More importantly, larger body size in rats allows for harvesting ample biological samples, enabling a wide range of detailed downstream applications. In parallel, rats have long been used in pharmacological efficacy and toxicity assays, suggesting a substantial and robust dataset for the cross-reference. All these factors again underline immunodeficient rats as a valuable bioresource in advancing cancer, stem cell, transplantation research, and drug development, which positions them as one of the most widely used animal models in medical and life sciences studies.
Available Strains
We are currently providing three immunodeficient rat strains. These rats are bred in clean and dedicated facilities to ensure that they are safe and suitable for experimental use. If you have any questions regarding breeding methods or facility requirements, please feel free to contact us.
| NBRP Rat No. | Strain | Characters | Genotyping* |
|---|---|---|---|
| 0883 | F344-Il2rgem1Iexas | A rat with a 5 bp deletion in the Il2rg gene on the X chromosome, resulting in severe combined immunodeficiency (SCID). The rat develops about as normally under SPF (Specific Pathogen-Free) conditions. | (Target) Il2rg PCR (Product Size) Wild Type : 292 bp, Mutant : 287 bp, (Primers) F: TTGCTGACTTCTATGGACCTTAAA, R: TTCATCTGGTCTGAACTGATAACTTAT |
| 0894 | F344-Rag2em1Iexas | A rat with a 1 bp insertion in the Rag2 gene on chromosome 3, exhibiting a phenotype similar to severe combined immunodeficiency (SCID). The rat develops about as normally under SPF (Specific Pathogen-Free) conditions. | (Target) Rag2 PCR (Product Size) Wilde type : 381bp, Mutant : 382bp, (Primers) F: GGGGAGAAGGTGTCTTACGG, R: AGGTGGGAGGTAGCAGGAAT |
| 0895 | F344-Il2rg/Rag2em1Iexas | A rat with a 5 bp deletion in the Il2rg gene and a 1 bp insertion in the Rag2 gene, resulting in severe combined immunodeficiency (SCID). It develops almost normally under SPF conditions, yet sexual maturation is slightly delayed. | Can be identified from the combined assay method of NBRP Rat No.0883 and 0894. |
Achievements
The fourth phase of NBRP-Rat began in April 2017, and the rat strain provision service has been recorded since then from Osaka University. The record and provision service continues even after the transfer to the University of Tokyo in April 2020.
| Fiscal Years | Number of Cases | Number of provided institutes | Number of provided rats |
|---|---|---|---|
| 2024 | 60 | 14 | 302 |
| 2023 | 49 | 13 | 304 |
| 2022 | 45 | 11 | 291 |
| 2021 | 22 | 11 | 91 |
| 2020 | 20 | 7 | 107 |
| 2019 | 15 | 6 | 60 |
| 2018 | 15 | 6 | 54 |
| 2017 | 1 | 1 | 14 |
Application Examples
List of Publications
Rat polyomavirus 2 infection: secondary publication. *NEW*
Tanaka M.
Exp Anim. 2026 Jan 1;75(1):1-9. doi: 10.1538/expanim.25-0072. Epub 2025 Sep 3. PMID: 40903308.
Dynamic assessment of a humanized bone tumour microenvironment reveals insights into osteosarcoma primary tumour remodelling and lung metastases. *NEW*
Gospos J, Laubach M, Savi FM, Ravichandran A, Bauer J, Friedrich O, Holzapfel BM, Wagner F, Mashimo T, Saifzadeh S, Hutmacher DW, McGovern JA.
Sci Rep. 2025 Nov 28;15(1):45619. doi: 10.1038/s41598-025-29941-z.
Genome editing using type I-E CRISPR-Cas3 in mice and rat zygotes.
Yoshimi K, Kuno A, Yamauchi Y, Hattori K, Taniguchi H, Mikamo K, Iida R, Ishida S, Goto M, Takeshita K, Ito R, Takahashi R, Takahashi S, Mashimo T.
Cell Reports Methods. 2024 August 19. doi: 10.1016/j.crmeth.2024.100833.
A rat-based preclinical platform facilitating transcatheter hepatic arterial infusion in immunodeficient rats with liver xenografts of patient-derived pancreatic ductal sdenocarcinoma.
Ozaki M, Kageyama K, Kimura K, Eguchi S, Yamamoto A, Tanaka R, Nota T, Yonezawa H, Nishiofuku H, Sakai Y, Tani N, Jogo A, Terai M, Sato T, Ishizawa T, Miki Y.
Scientific Reports. 2024 May 2. doi: 10.1038/s41598-024-61142-y.
Immune deficiency phenotypes of Il2rg, Rag2 or Il2rg/Rag2 double knockout rats; establishment of human leukemia xenograft models. Kim JI, Lim HJ, Kwon E, Mashimo T, Kang BC. Lab Anim Res. 2024 Dec 27;40(1):43. doi: 10.1186/s42826-024-00231-5.
Human iPSC-liver organoid transplantation reduces fibrosis through immunomodulation.
Tadokoro T, Murata S, Kato M, Ueno Y, Tsuchida T, Okumura A, Kuse Y, Konno T, Uchida Y, Yamakawa Y, Zushi M, Yajima M, Kobayashi T, Hasegawa S, Kawakatsu-Hatada Y, Hayashi Y, Osakabe S, Maeda T, Kimura K, Mori A, Tanaka M, Kamishibahara Y, Matsuo M, Nie YZ, Okamoto S, Oba T, Tanimizu N, Taniguchi H.
Sci Transl Med. 2024 Jul 24;16(757):eadg0338. doi: 10.1126/scitranslmed.adg0338. Epub 2024 Jul 24.
A novel Kit mutant rat enables hematopoietic stem cell engraftment without irradiation.
Iida R, Ishida S, Wang J, Hattori K, Yoshimi K, Yamazaki S, Mashimo T.
Exp Hematol. 2024 Feb 6. doi: 10.1016/j.exphem.2024.104174.
Comparative study of immunodeficient rat strains in engraftment of hiPSC-derived airway epithelia.
Hayashi Y, Ohnishi H, Kitano M, Kishimoto Y, Takezawa T, Okuyama H, Yoshimatsu M, Kuwata F, Tada T, Mizuno K, Omori K.
Tissue Eng Part A. 2023 Nov 11. doi: 10.1089/ten.TEA.2023.0214. Online ahead of print.
A high-quality severe combined immunodeficiency (SCID) rat bioresource.
Miyasaka Y, Wang J, Hattori K, Yamauchi Y, Hoshi M, Yoshimi K, Ishida S, Mashimo T.
PLoS One. 2022 Aug 12;17(8):e0272950. doi: 10.1371/journal.pone.0272950. eCollection 2022. PMID: 35960733.
Optimal Organ for Patient-derived Xenograft Model in Pancreatic Cancer and Microenvironment that Contributes to Success.
Eguchi S, Kimura K, Kageyama K, Tani N, Tanaka R, Nishio K, Shinkawa H, Ohira GO, Amano R, Tanaka S, Yamamoto A, Takemura S, Yashiro M, Kubo S.
Anticancer Res. 2022 May;42(5):2395-2404. doi: 10.21873/anticanres.15718.
Transplantation of a human induced pluripotent stem cell-derived airway epithelial cell sheet into the middle ear of rats.
Tada T, Ohnishi H, Yamamoto N, Kuwata F, Hayashi Y, Okuyama H, Morino T, Kasai Y, Kojima H, Omori K.
Regen Ther. 2022 Jan 14;19:77-87. doi: 10.1016/j.reth.2022.01.001. eCollection 2022 Mar. PMID: 35097166.
A humanised rat model reveals ultrastructural differences between bone and mineralised tumour tissue.
Lahr CA, Landgraf M, Wagner F, Cipitria A, Moreno-Jiménez I, Bas O, Schmutz B, Meinert C, Mashimo T, Miyasaka Y, Holzapfel BM, Shafiee A, McGovern JA, Hutmacher DW.
Bone. 2021 May 20:116018. doi: 10.1016/j.bone.2021.116018. Online ahead of print.
In vivo regeneration of rat laryngeal cartilage with mesenchymal stem cells derived from human induced pluripotent stem cells via neural crest cells.
Yoshimatsu M, Ohnishi H, Zhao C, Hayashi Y, Kuwata F, Kaba S, Okuyama H, Kawai Y, Hiwatashi N, Kishimoto Y, Sakamoto T, Ikeya M, Omori K.
Stem Cell Res. 2021 Apr;52:102233. doi: 10.1016/j.scr.2021.102233. Epub 2021 Feb 11.
Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells.
Takeishi K, Collin de l'Hortet A, Wang Y, Handa K, Guzman-Lepe J, Matsubara K, Morita K, Jang S, Haep N, Florentino RM, Yuan F, Fukumitsu K, Tobita K, Sun W, Franks J, Delgado ER, Shapiro EM, Fraunhoffer NA, Duncan AW, Yagi H, Mashimo T, Fox IJ, Soto-Gutierrez A.
Cell Rep. 2020 Jun 2;31(9):107711.
Zonisamide promotes survival of human-induced pluripotent stem cell-derived dopaminergic neurons in the striatum of female rats.
Miyawaki Y, Samata B, Kikuchi T, Nishimura K, Takahashi J.
J Neurosci Res. 2020 Aug;98(8):1575-1587.
Intestinal immunity suppresses carrying capacity of rats for the model tapeworm, Hymenolepis diminuta.
Ohno T, Kai T, Miyasaka Y, Maruyama H, Ishih A, Kino H.
Parasitol Int. 2018 Aug;67(4):357-361.
Fail-safe therapy by gamma-ray irradiation against tumor formation by human induced pluripotent stem cell-derived neural progenitors.
Katsukawa M, Nakajima Y, Fukumoto A, Doi D, Takahashi J.
Stem Cells Dev. 2016 Jun 1;25(11):815-825.
Estradiol Facilitates Functional Integration of iPSC-Derived Dopaminergic Neurons into Striatal Neuronal Circuits via Activation of Integrin α5β1.
Nishimura K, Doi D, Samata B, Murayama S, Tahara T, Onoe H, Takahashi J.
Stem Cell Reports. 2016 Apr 12;6(4):511-524.
Generation of Scaffoldless Hyaline Cartilaginous Tissue from Human iPSCs.
Yamashita A, Morioka M, Yahara Y, Okada M, Kobayashi T, Kuriyama S, Matsuda S, and Tsumaki N.
Stem Cell Reports. 2015 Mar 10;4(3):404-418.
X-linked severe combined immunodeficiency (X-SCID) rats for xeno-transplantation and behavioral evaluation.
Samata B, Kikuchi T, Miyawaki Y, Morizane A, Mashimo T, Nakagawa M, Okita K, Takahashi J.
J Neurosci Methods. 2015 Mar 30;243:68-77.
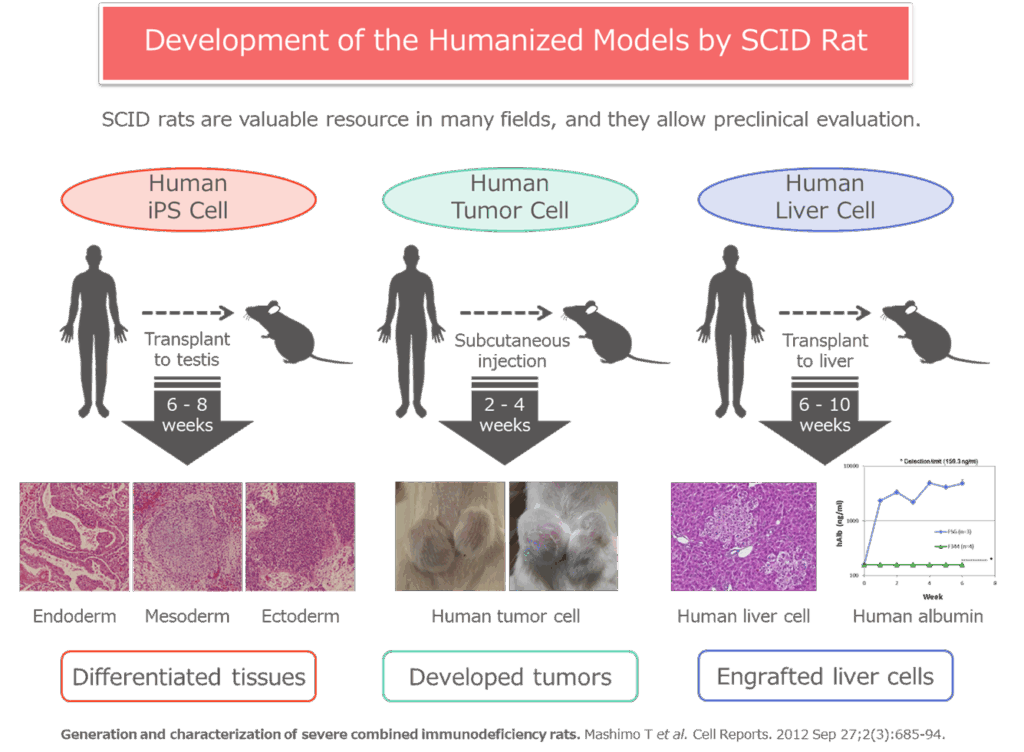
Generation and characterization of severe combined immunodeficiency rats.
Mashimo T, Takizawa A, Kobayashi J, Kunihiro Y, Yoshimi K, Ishida S, Tanabe K, Yanagi A, Tachibana A, Hirose J, Yomoda J, Morimoto S, Kuramoto T, Voigt B, Watanabe T, Hiai H, Tateno C, Komatsu K, Serikawa T.
Cell Reports. 2012 Sep 27;2(3):685-694.
Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases.
Mashimo T, Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T.
PLoS One. 2010 Jan 25;5(1):e8870.